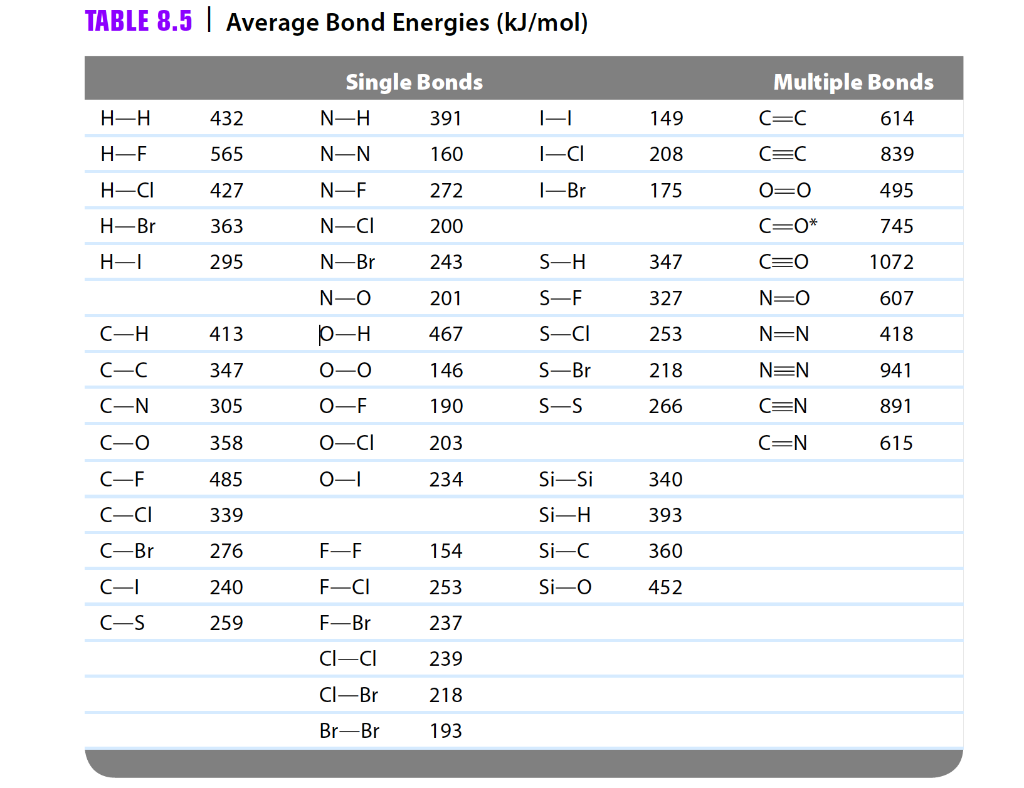
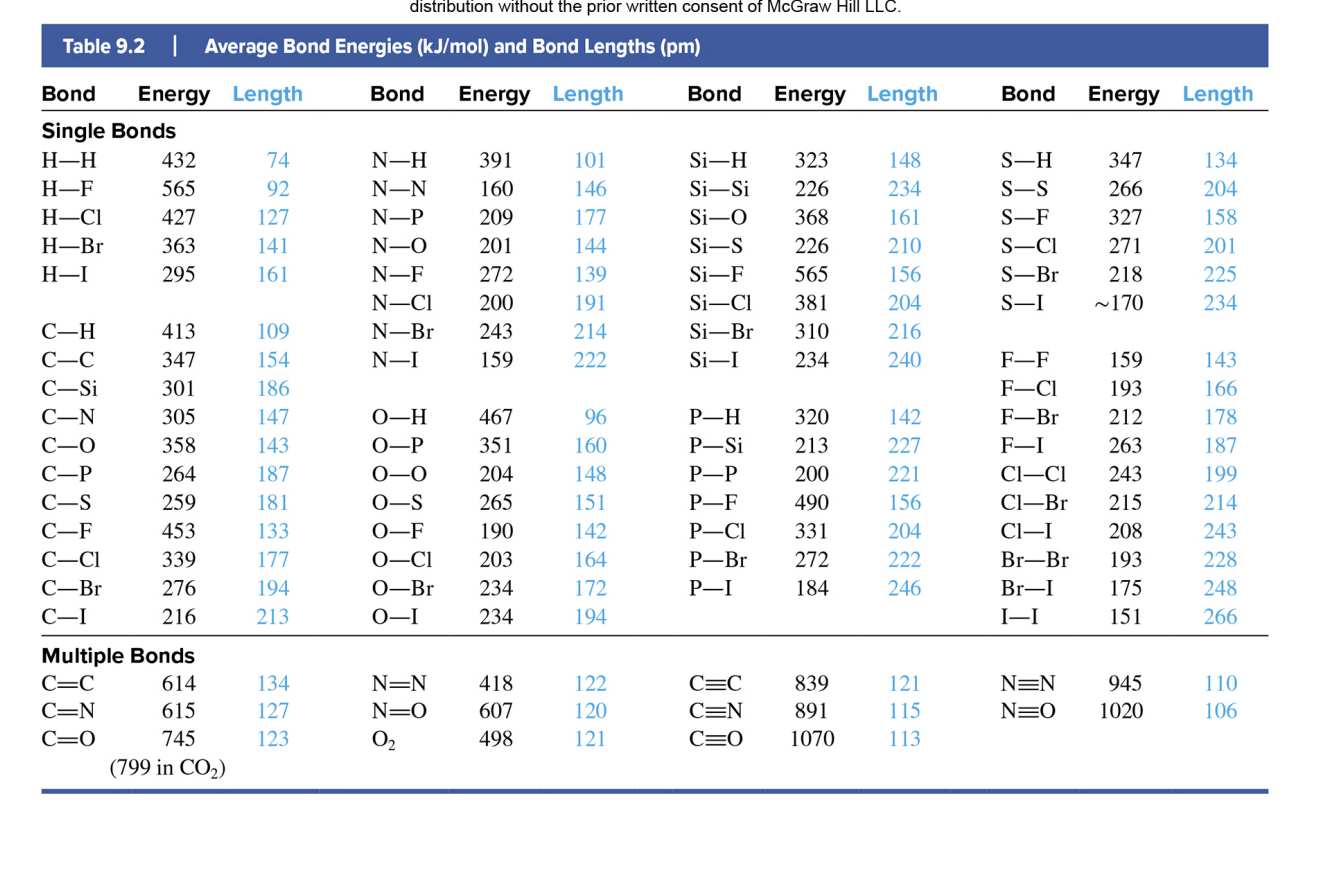
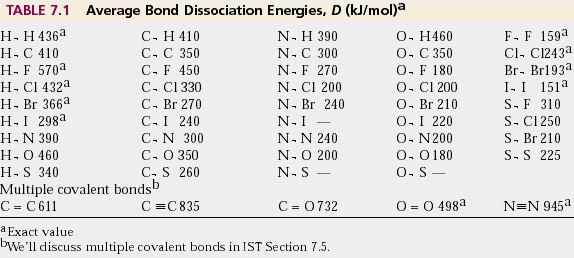
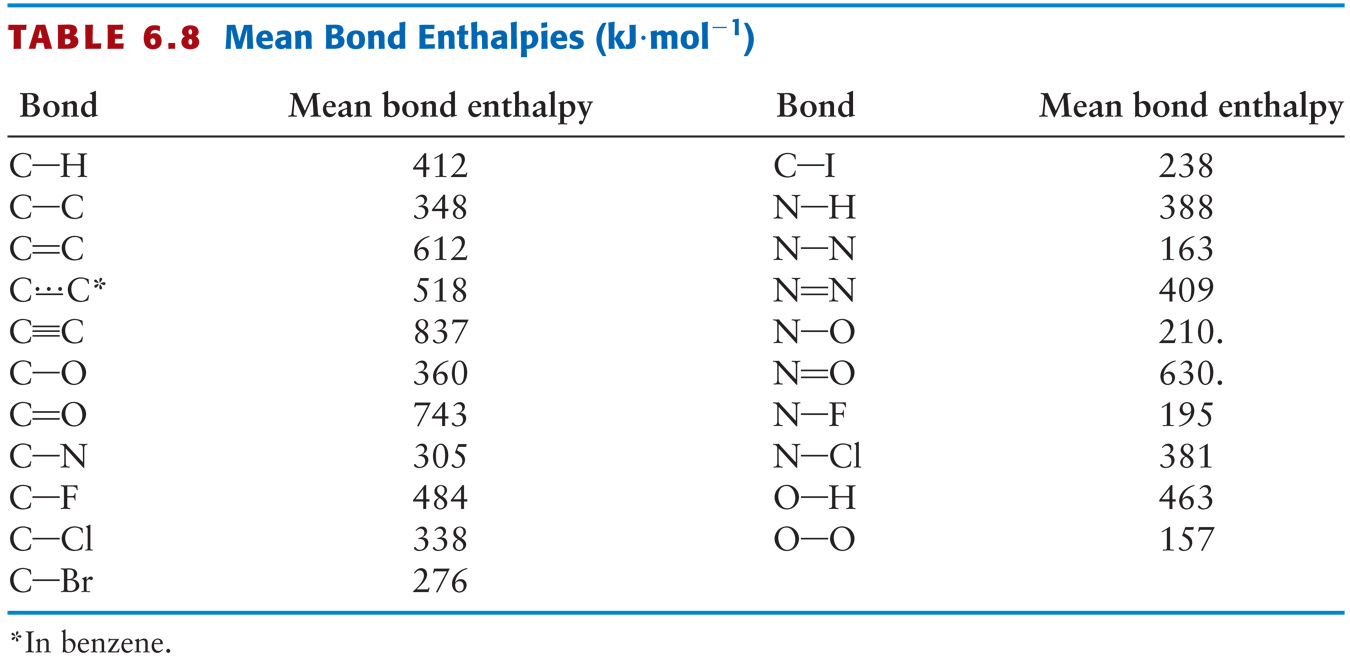
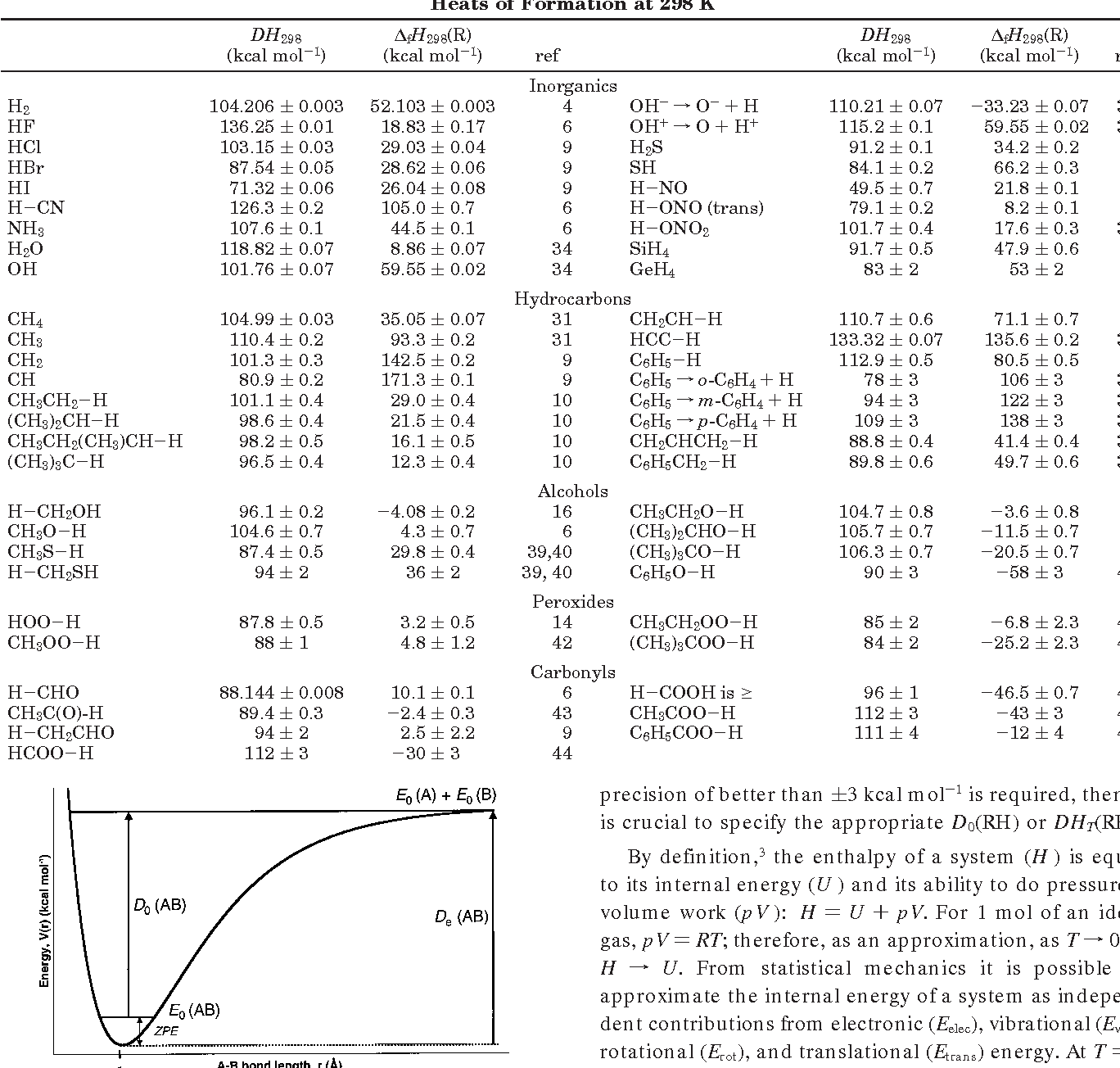
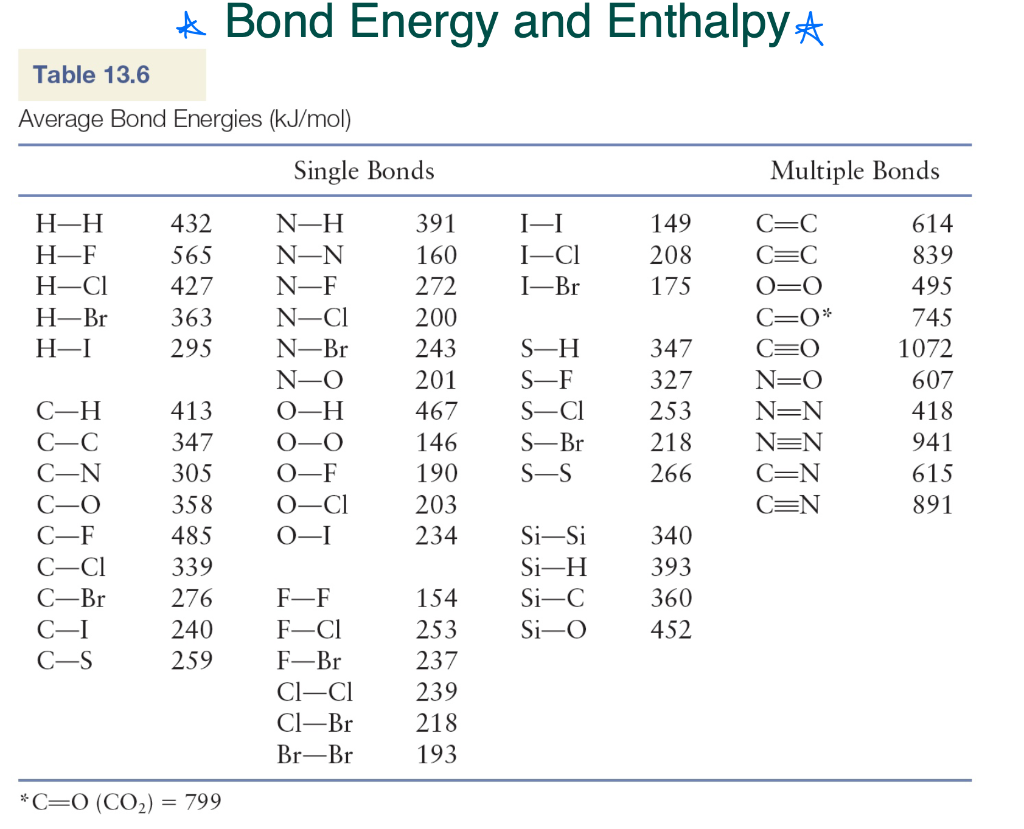
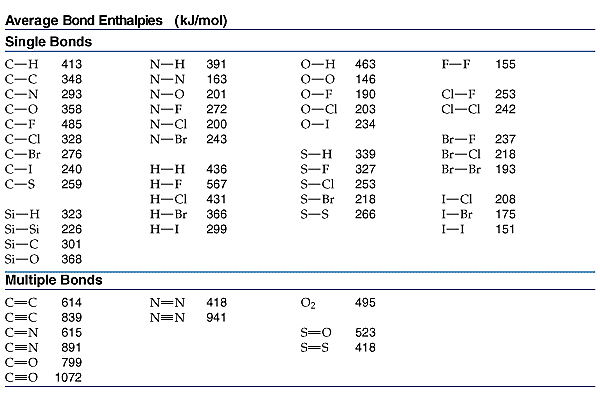
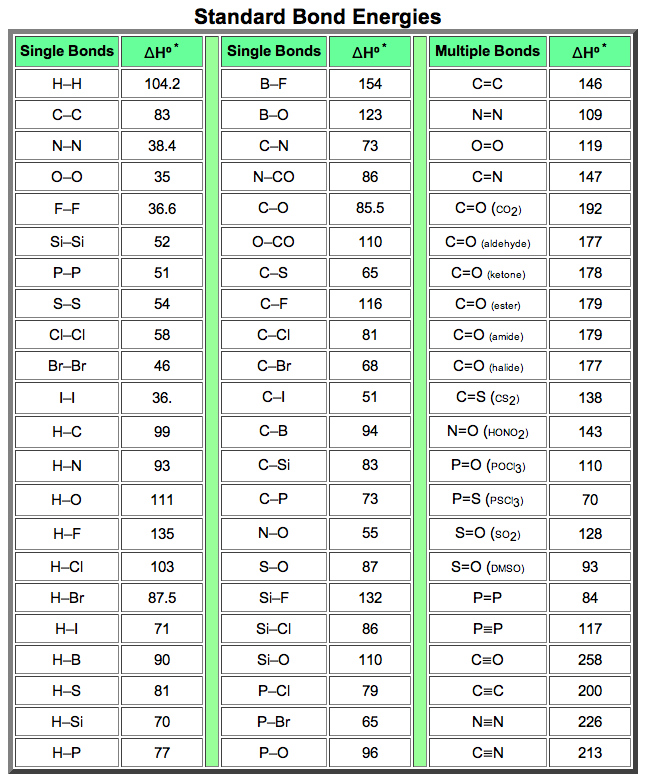
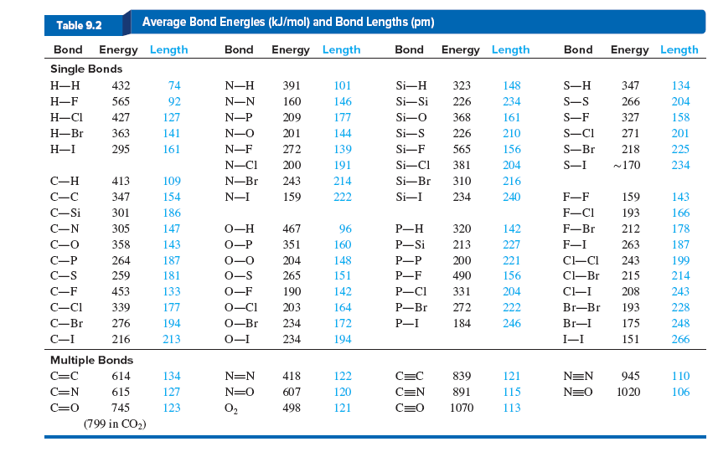
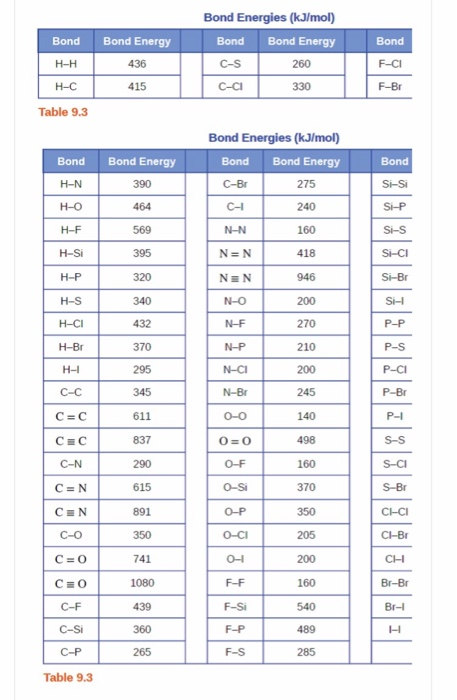
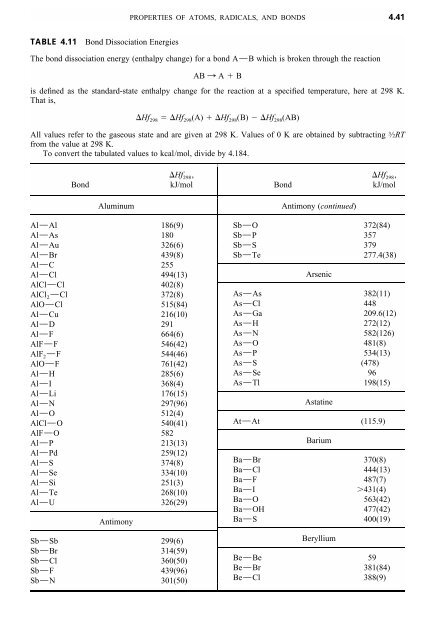
Bond Energy Table.pdf - Bond Energies Bond H-H H-C H-N H-O H-S H-F H-Cl H-Br H-I BE kJ | Course Hero
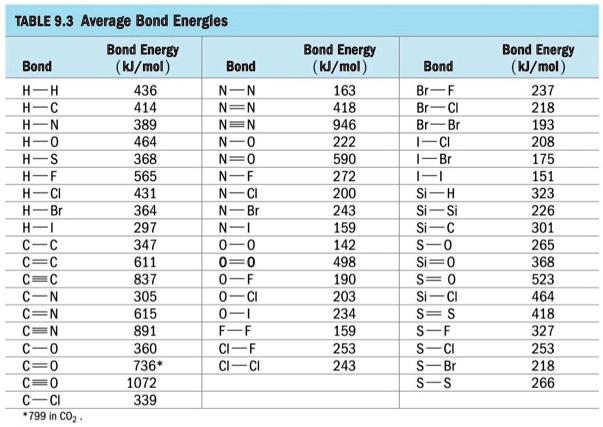
SOLVED: TABLE 9.3 Average Bond Energies Bond Energy (kJ/mol) Bond 436 Bond 414 Bond 389 Bond Energy (kJ/mol) Bond 237 Bond 218 Bond 193 Bond 208 Bond 175 Bond 151 Bond 228
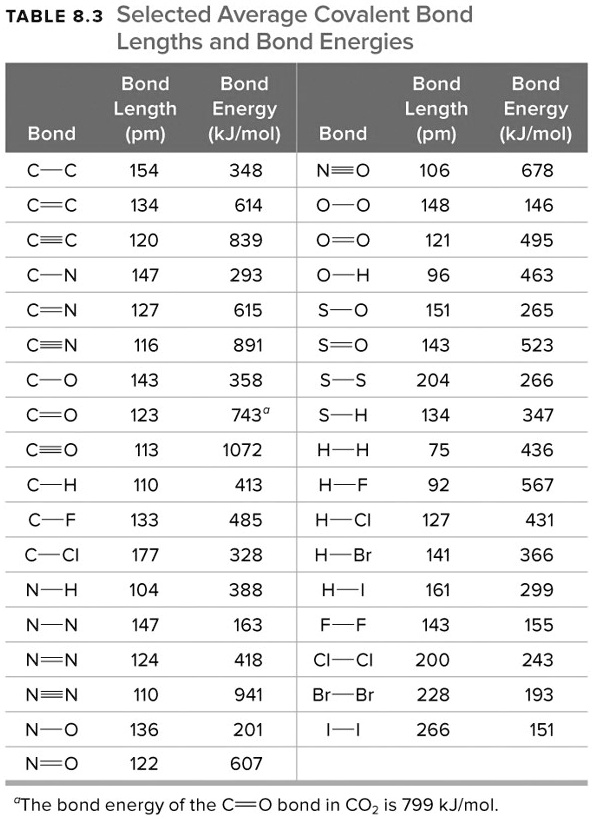
SOLVED: TABLE 8.3 Selected Average Covalent Bond Lengths and Bond Energies Bond Length (pm) Bond Energy (kJ/mol) C-C 348 C=C 106 678 C=N 154 N=O 134 C=O 614 C-F 148 C-Cl 146