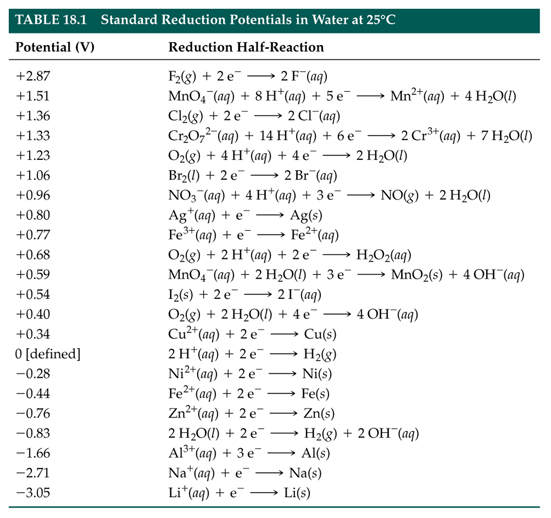
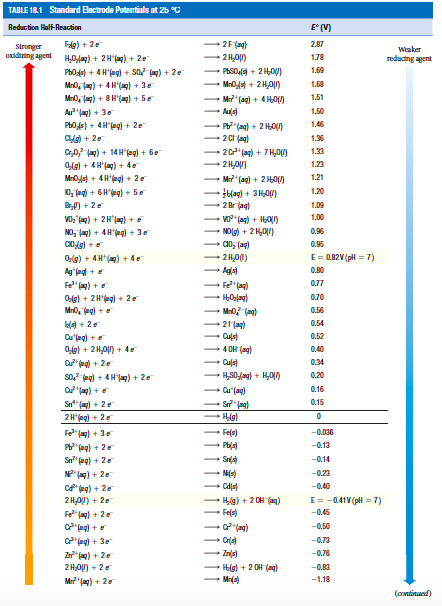
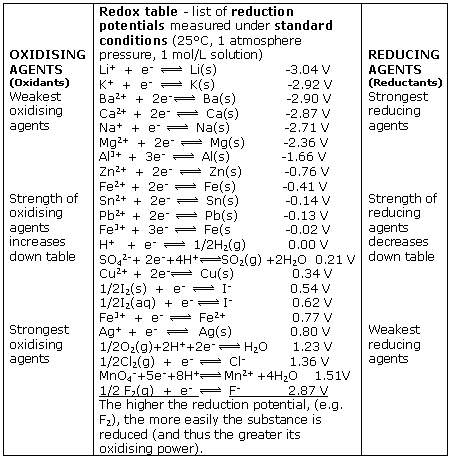
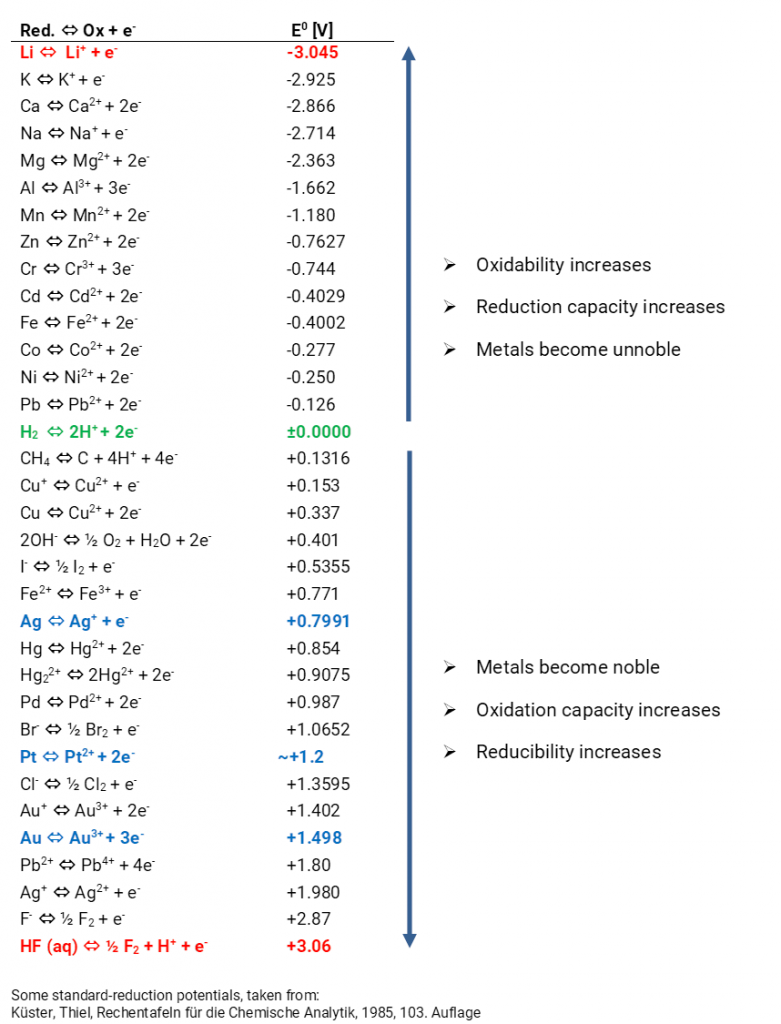
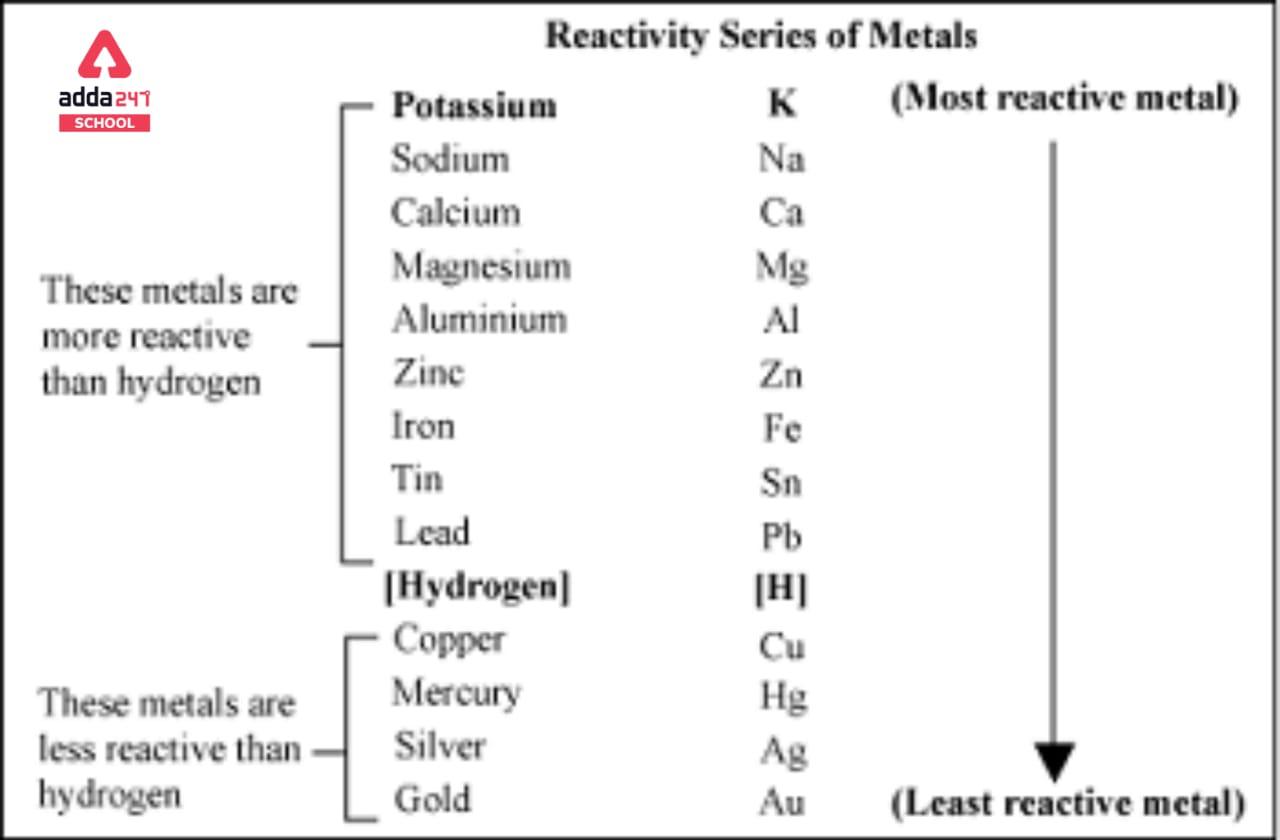
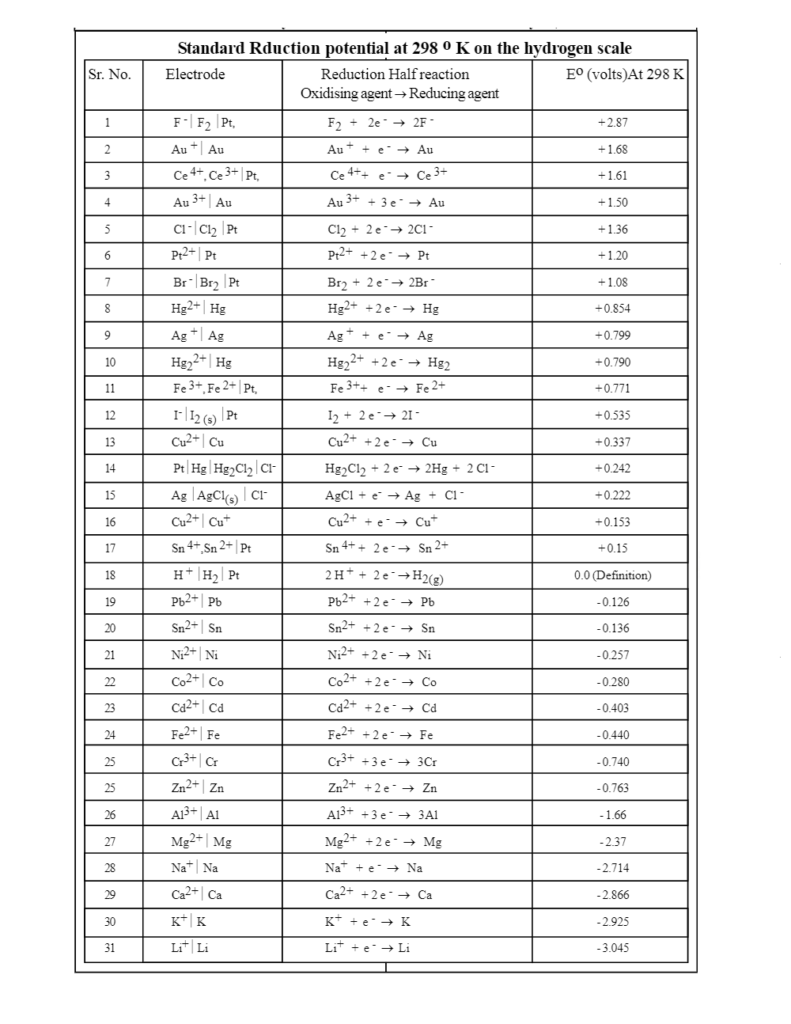
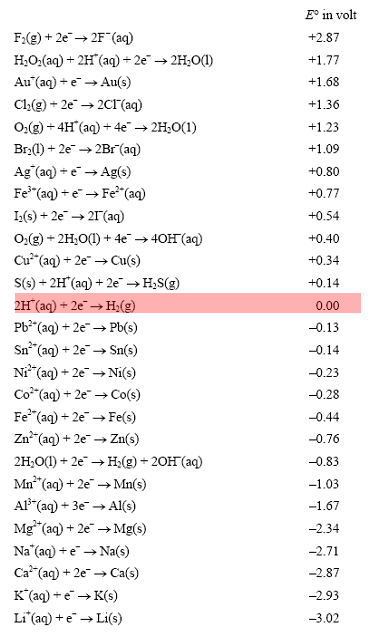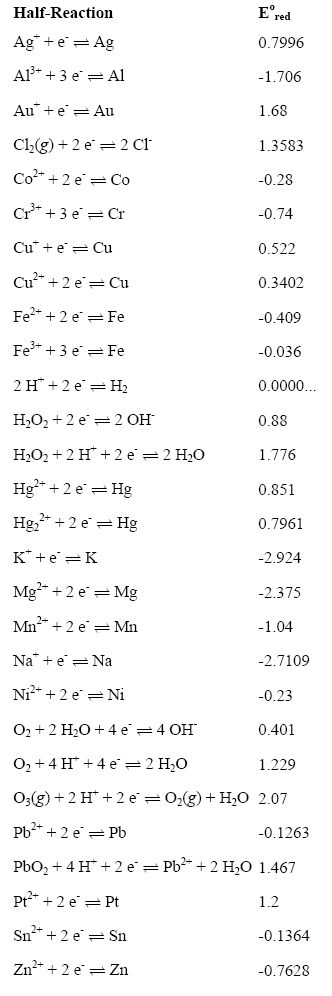![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)
PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar

SOLVED: Table 1: Electrochemical Cell Potentials Individual Half-Cell Potentials Measured Total Potential from Multimeter Metal Metal Electrode Electrode Experimental Theoretical Potential (V)? Potential (vn Metal Electrode Potential % Error Cell Cu ...

Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25°C | Semantic Scholar