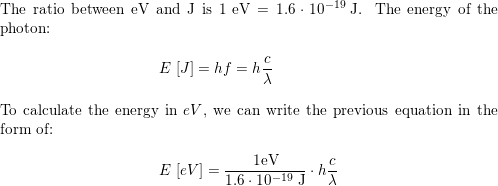Electric Vehicle Conversion Kits 145kw 155kwkw 300nm 310nm Electric Car Conversion Kit 2t 2.5t Complete Kit for Automotive Electric Motor - China Electric Vehicle Motor, AC Induction Motor | Made-in-China.com

Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download

Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download
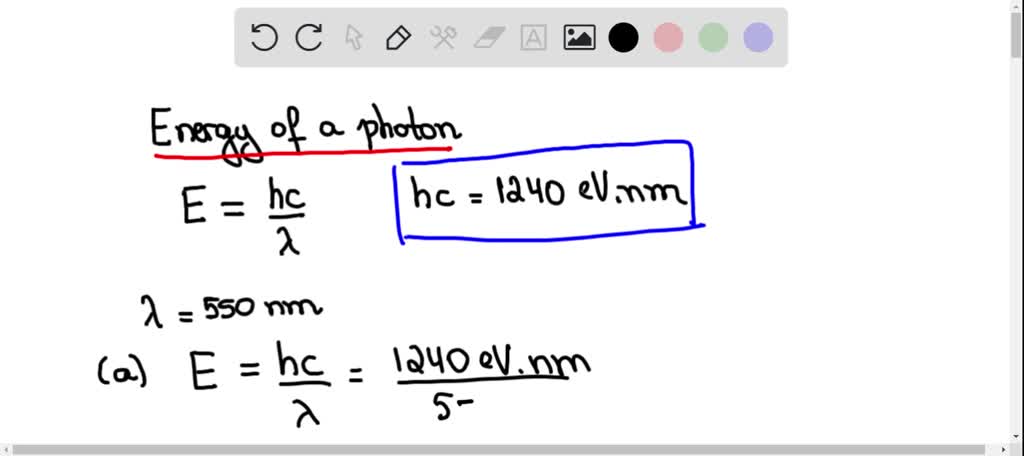
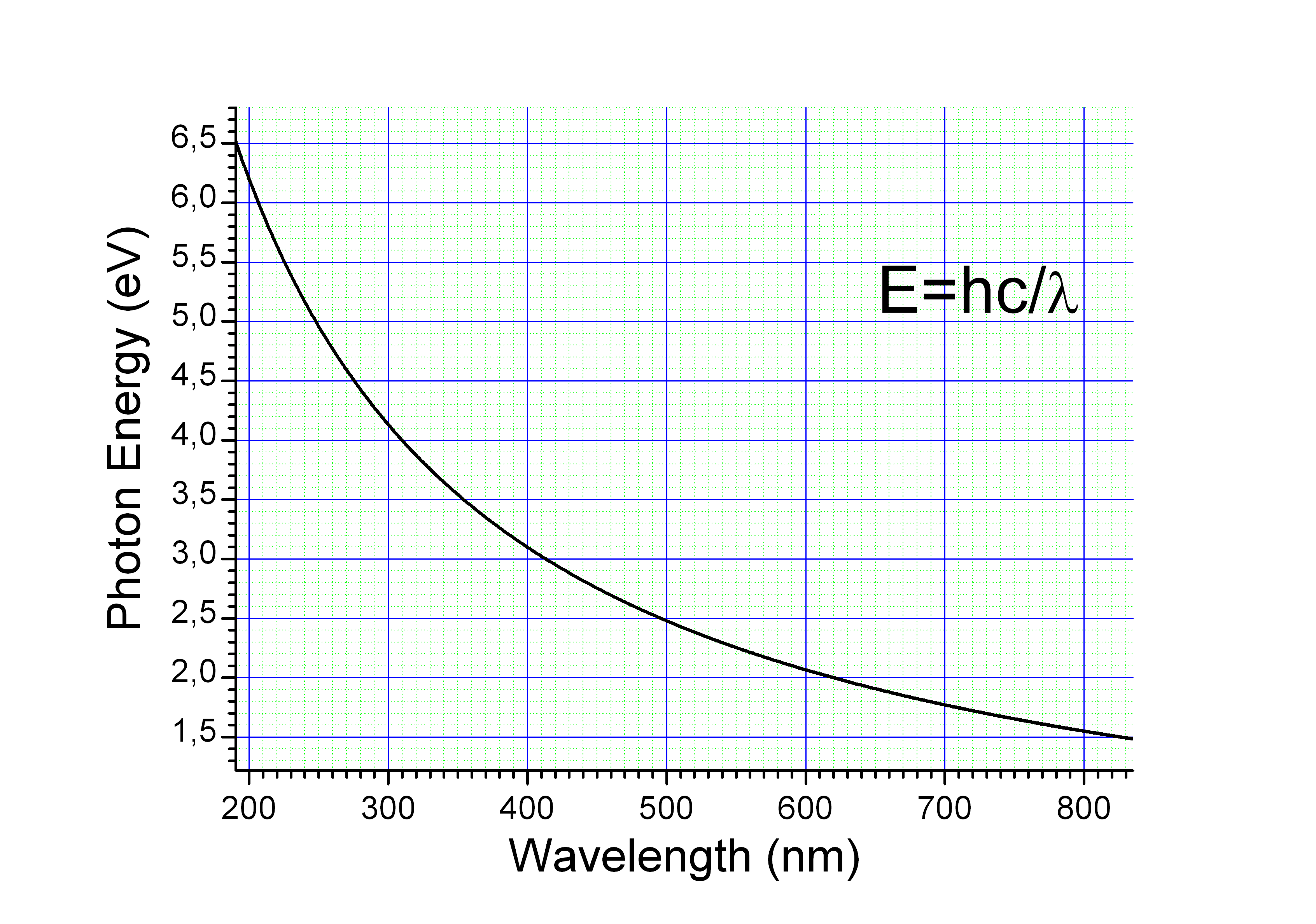
A firefly glows by the direct conversion of chemical energy to light. The light emitted by a firefly has peak intensity at a wavelength of 550 nm a. What is the minimum chemical energy, in eV, ...
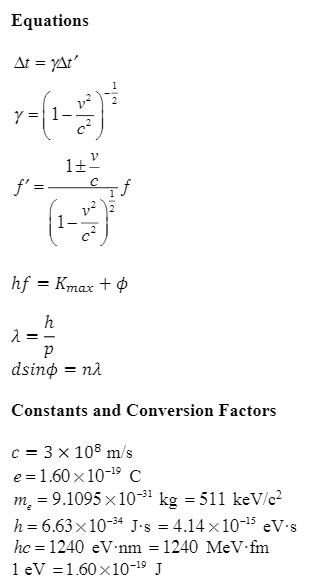
SOLVED: Equations Kt = YAt' 7-(1-7) 1+" f' (f' hf Kmar + 1 = dsinθ = nl Constants and Conversion Factors c = 3x108 m/s e = 1.60x10-19 C m = 9.1095x10-31

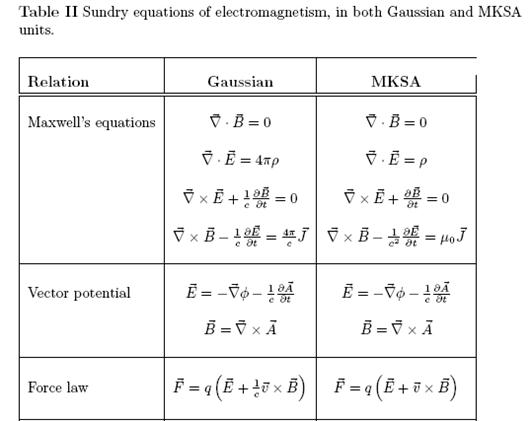
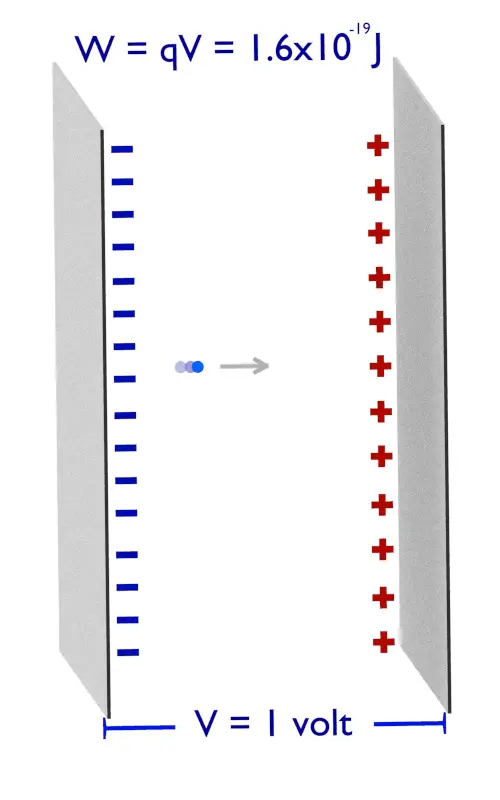
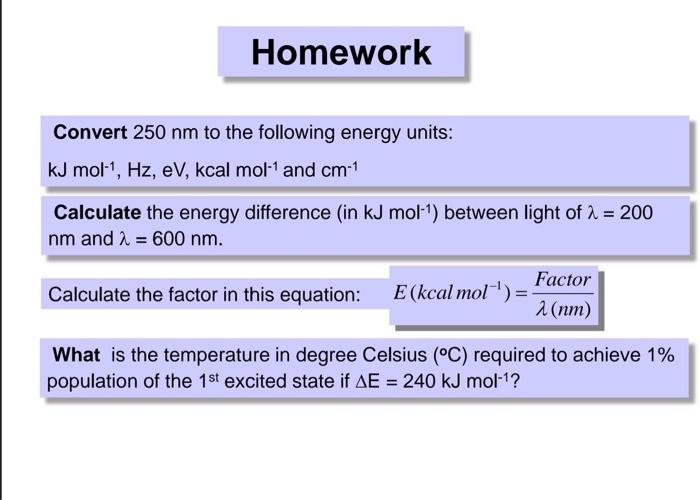
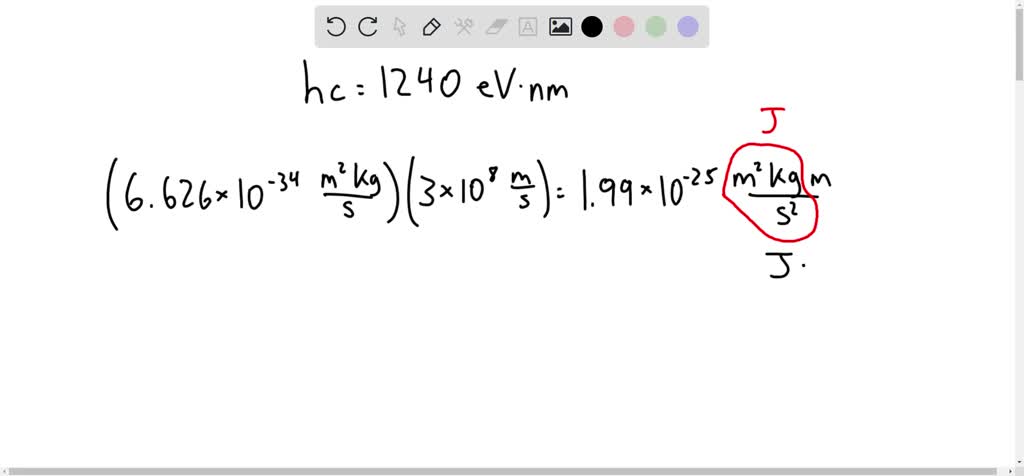
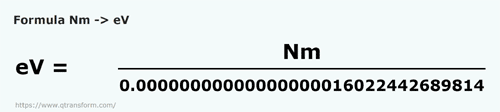
Derivation of hc=1240eVnm from SI units to eVnm + compute the energy of the Lyman-alpha photon in eV
a) Down-conversion (DC) PL spectra at an excitation of 450 nm for the... | Download Scientific Diagram